Antimicrobial activity of essential oil from Mimosa verrucosa Benth. and Illicium verum Hook.f. against planktonic and biomass bacterial cells
DOI:
https://doi.org/10.70151/1vw7fj54Keywords:
microbiological resistance, monoterpene, phenylpropanoids, volatile oilsAbstract
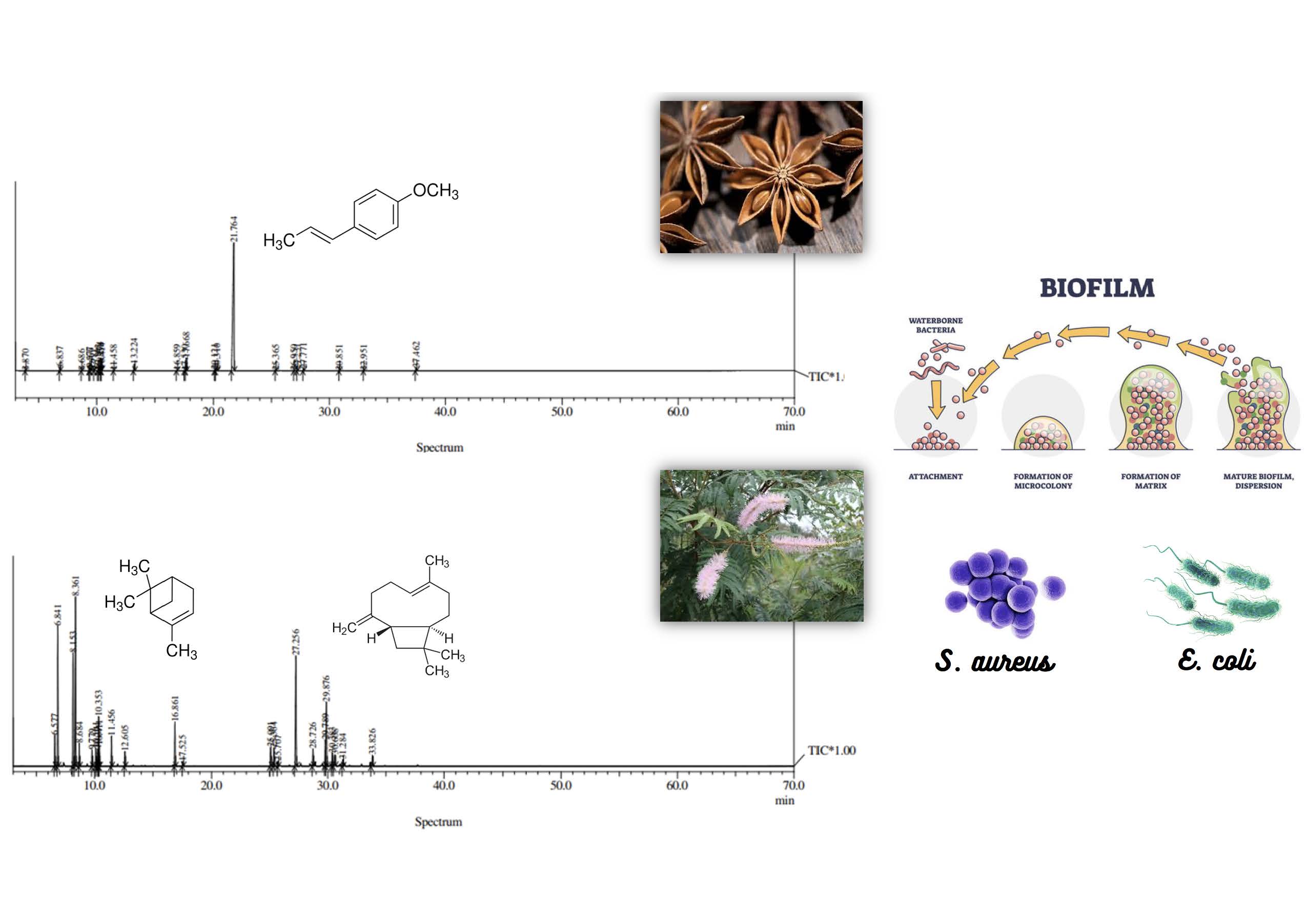
The development of biofilms is responsible for 80% of microbial infections, which are highly resistant to conventional antibiotics. The study aimed to assess the antimicrobial activity of essential oils of Mimosa verrucosa Benth. and Illicium verum Hook.f. against planktonic and sessile cells and evaluate toxicity. Thirty compounds were identified, the main ones being a-pinene (12.6%), b-pinene (16.7%) and (E)-caryophyllene (14.2%) for M. verrucosa, and estragole (4.2%) and anethole (86.8%) for I. verum. The minimum inhibitory concentrations of the EO of I. verum (29.40 µg/ml) and M. verrucosa (24.89 µg/ml) against planktonic cells showed 99% efficacy against all cells tested (sensitive and resistant Staphylococcus aureus and Escherichia coli). In sessile cells, essential oils of I. verum and M. verrucosa showed efficacy against sensitive S. aureus. The minimum bactericidal concentration (MBC) test revealed that I. verum caused cell death in sensitive S. aureus and E. coli. However, M. verrucosa only showed bactericidal activity against planktonic cells. Considering the expanding resistance to antimicrobials, the EOs tested represent an important therapeutic option, especially against S. aureus and E. coli, which can produce biofilms on various surfaces, becoming a serious public health problem.
Downloads
References
Abirami SG, Mani KS, Devi MN, Devi PN (2014) The antimicrobial activity of Mimosa pudica L. Int J Ayur Pharma Res 2(1):1-4. https://ijapr.in/index.php/ijapr/article/view/289
Adams, RP (2007) Identification of essential oil components by gas chromatography/ mass spectrometry, 4th ed. Allured Publ., Carol Stream. 804p.
Albano M, Crulhas BP, Alves FCB, Pereira AFM, Andrade BFMT (2019) Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb Pathog 126:231–238. https://doi.org/10.1016/j.micpath.2018.11.009
BrCast – Brazilian Committee on Antimicrobial Susceptibility Testing, 2023. in: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recom mendations_for_MIC_determination_of_colistin_March_2016.pdf.
Bussmann RW, Malca G, Glenn A, Sharon D, Nilsen B, Parris B, Dubose D, Ruiz D, Saleda J, Martinez M, Carillo L, Walker K, Kuhlman A, Townesmith A (2011) Toxicity of medicinal plants used in traditional medicine in Northern Peru. J Ethnopharmacol 137(1):121-40. https://doi.org/10.1016/j.jep.2011.04.071
Cerca N, Azevedo NF (2012) Biofilmes na saúde, no meio ambiente, na indústria, Publindústria, 1st ed. Braga, Porto. 398p.
Condò C, Anacarso I, Sabia C, Iseppi R, Anfelli I, Forti L, Niederhäusern S, Bondi M, Messi P (2020) Antimicrobial activity of spices essential oils and its effectiveness on mature biofilms of human pathogens. Nat Prod Res 34(4):567-574. https://doi.org/10.1080/14786419.2018.1490904
Davey ME, O'toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64(4):847-67. https://doi.org/10.1128/MMBR.64.4.847-867.2000
de Souza Araújo E, Pimenta AS, Feijó FMC, Castro RVO, Fasciotti M (2018) Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J Appl Microbiol 124(1):85-96. https://doi.org/10.1111/jam.13626
de Souza ERP, Braz MVDC, Castro RN, Pereira MD, Riger CJ (2023) Influence of microbial fermentation on the antioxidant activity of phenolic substances in Saccharomyces cerevisiae. J Appl Microbiol 134(8):lxad148. https://doi.org/10.1093/jambio/lxad148
Dos Santos Silva RA, Calumby RJN, da Silva Santos IK, da Silva SAS, de Almeida LM, Nascimento TG, Junior IDB (2020) Technological prospection of Antibacterial and antifungal Potential of star anise (Illicium verum Hook.f.). Rev. Humanid Inov 7(4): 327-338.
Epifanio NMM, Cavalcanti LRI, Dos Santos KF, Duarte PSC, Kachlicki P, Ożarowski M, Riger CJ, Chaves DAS (2020) Chemical characterization and in vivo antioxidant activity of parsley (Petroselinum crispum) aqueous extract. Food Funct 11(6):5346-5356. https://doi.org/10.1039/d0fo00484g
Franco CJP, Ferreira OO, Cruz JN, Varela ELP, de Moraes ÂAB, Nascimento LD, Cascaes MM, Souza Filho APDS, Lima RR, Percário S (2022) Phytochemical profile and herbicidal (phytotoxic), antioxidants potential of essential oils from Calycolpus goetheanus (Myrtaceae) specimens, and in silico study. Molecules 27(15):4678. https://doi.org/10.3390/molecules27154678
Gajewska J, Chajęcka-Wierzchowska W (2020) Biofilm formation ability and presence of adhesion genes among coagulase-negative and coagulase-positive staphylococci isolates from Raw Cow's Milk. Pathogens (8):654. https://doi.org/10.3390/pathogens9080654
Ganesh PS, Veena K, Senthil R, Iswamy K, Ponmalar EM, Mariappan V, Girija SS, Vadivelu J, Nagarajan S, Challabathula D, Shankar EM (2022) Biofilm-associated agr and sar quorum sensing systems of Staphylococcus aureus are inhibited by 3-hydroxybenzoic acid derived from Illicium verum. ACS Omega 7(17):14653-14665. https://doi.org/10.1021/acsomega.1c07178
Ghazal TSA, Schelz Z, Vidács L, Szemerédi N, Veres K, Spengler G, Hohmann J (2022) Antimicrobial, multidrug resistance reversal and biofilm formation inhibitory effect of Origanum majorana extracts, essential oil and monoterpenes. Plants 11(11):1432. https://doi.org/10.3390/plants11111432
Goetghebeur M, Landry PA, Han D, Vicente C (2007) Methicillin-resistant Staphylococcus aureus: A public health issue with economic consequences. Can J Infect Dis Med Microbiol (1):27-34. https://doi.org/10.1155/2007/253947
Jain S, Jain R, Vlietinck A (2004) In vivo and in vitro antimicrobial efficacy of Mimosa hamata. Indian J Biotech 3:271–273.
Jiang T, Li, M (2013) Quorum sensing inhibitors: a patent review. Expert Opin Ther Pat 7:867–894. https://doi.org/10.1517/13543776.2013.779674
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4(12):e01067. https://doi.org/10.1016/j.heliyon.2018.e01067
Majeed I, Rizwan K, Ashar A, Rasheed T, Amarowicz R, Kausar H, Zia-Ul-Haq M, Marceanu LG (2021) A comprehensive review of the ethnotraditional uses and biological and pharmacological potential of the genus Mimosa. Int J Mol Sci 22(14):7463. https://doi.org/10.3390/ijms22147463
Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H (2011) Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol 77(22):8097-105. https://doi.org/10.1128/AEM.05316-11
Ortega E, Abriouel H, Lucas R, Gálvez A (2010) Multiple roles of Staphylococcus aureus enterotoxins: pathogenicity, superantigenic activity, and correlation to antibiotic resistance. Toxins 2(8):2117-31. https://doi.org/10.3390/toxins2082117
Outemsa B, Oubihi A, Jaber H, Haida S, Kenfaoui I, Ihamdan R, Azhari HA, Ouhssine M (2021) Chemical composition, antioxidant and antimicrobial activities of the essential oil of Illicium verum. E3S Web Conf 319: 01052-01059. https://doi.org/10.1051/e3sconf/202131901052
Padilha IQM, Pereira AV, Rodrigues OG, Siqueira-Junior JP, Pereira MSV (2010) Antimicrobial activity of Mimosa tenuiflora (Willd.) Poir. from Northeast Brazil against clinical isolates of Staphylococcus aureus. Rev Bras Farmacogn 20(1):45-47. https://doi.org/10.1590/S0102-695X2010000100010
Romanoski VS, Santos RAF (2017) Cytotoxic and antioxidant activity of Mimosa verrucosa Benth. Orbital: Electron J Chem 9:100-104. https://doi.org/10.17807/orbital.v9i2.868
Silva SANM, Barros AB, Souza JMT, Moura AF, Araújo AR, Mendes MGA, Daboit TC, Silva DA, Araújo AJ, Filho JDBM (2020) Phytochemical and biological prospection of Mimosa genus plants extracts from Brazilian northeast. Phytochem Lett 39:173-181. https://doi.org/10.1016/j.phytol.2020.08.010
Villarreal SML, Luévano JHE, Hernández RAP, García ES, Star MJV, Ríos RC, Tapia MG, Luis OER, Montes AC (2022) Preliminary study of the antimicrobial, anticoagulant, antioxidant, cytotoxic, and anti-Inflammatory activity of five selected plants with therapeutic application in dentistry. Int J Environ Res Public Health. 19(13):7927. https://doi.org/10.3390/ijerph19137927
Wang GW, Hu WT, Huang BK, Qin LP (2011) Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol 136(1):10-20. https://doi.org/10.1016/j.jep.2011.04.051
Wang Q, Jiang L, Wen Q (2007) Effect of three extraction methods on the volatile component of Illicium verum Hook.f. analyzed by GC-MS. Wuhan Univ J Nat Sci 12(3):529-534. https://doi.org/10.1007/s11859-006-0080-7
Wińska K, Mączka W, Łyczko J, Grabarczyk M, Czubaszek A, Szumny A (2019) Essential oils as antimicrobial agents-myth or real alternative? Molecules 24(11):2130. https://doi.org/10.3390/molecules24112130
World Health Organization – WHO (2022) Global antimicrobial resistance and use surveillance system (GLASS) report: 2022. Available at: https://www.who.int/publications/i/item/9789240062702. Accessed on: 18 Jul 2024.
Yang EC, Hsieh YY, Chuang LY (2021). Comparison of the phytochemical composition and antibacterial activities of the various extracts from leaves and twigs of Illicium verum. Molecules 26(13):3909. https://doi.org/10.3390/molecules26133909
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

