Water suppression indicates the prevalence of the secondary defense system in Piper aduncum
DOI:
https://doi.org/10.70151/zq9m5a98Keywords:
Terpenes, phenylpropanoids, medicinal plants, water stress, essential oilAbstract
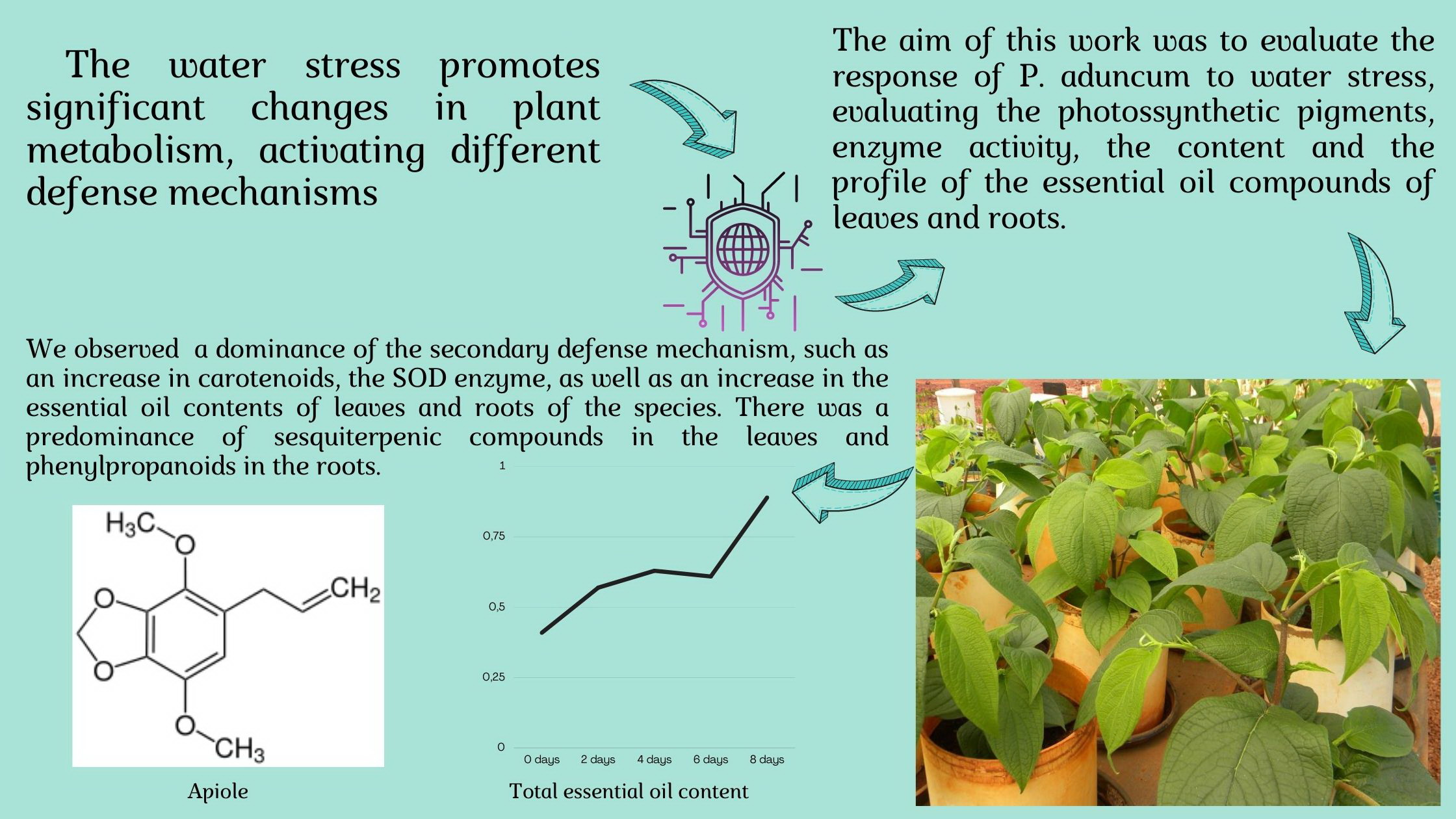
The aim of this work was to evaluate the response of Piper aduncum to water suppression. The experiment was conducted in a greenhouse, in an entirely randomized blocks, with five treatments: 0, 2, 4, 6, and 8 days without irrigation. After this period, dry matter, photosynthetic pigments (chlorophyll a, b and carotenoids), leaf temperature, activity of the enzymes ascorbate peroxidase (APX), catalase (CAT) and superoxide dismutase (SOD) were evaluated. The essential oil content of leaves and roots was also quantified through hydrodistillation, as well as the identification of constituents by CG-MS. The period of water suppression influenced the content of chlorophyll a, carotenoids and enzymatic activity of APX and CAT. The activities of APX and CAT were reduced under low water availability (CAT only increased after 4 days of suppression). Meanwhile, SOD had its activity increased under eight days of water suppression. In addition, there was an increase in essential oil content when subjected to stress. The predominant classes of constituents in the leaves were sesquiterpenes (32.56-36.54%) and phenylpropanoids (33.12-44.97%) in the roots. E-nerolidol was the major constituent of leaves (23.56-26.75%) and apiol (17.57-32.78%) of the roots. Thus, water suppression favors the secondary metabolism of the species.
Downloads
References
Abdelmajeed NA, Danial EN, Ayad HS (2013) The effect of environmental stress on qualitative and quantitative essential oil of aromatic and medicinal plants. Arch Sci 66:100-120.
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. 4.ed. Illinois: Allured Publish Corporation.804p.
Alvarenga ICA, Valadares RV, Martins ER, Oliveira FG, De Figueiredo LS, Kobayashi MK. (2011) Water stress before harvest of pepper-rosmarin. Pesqui Agropec Bras 46:706-711. http://dx.doi.org/10.1590/S0100-204X2011000700005
Alvarenga ICA, Pacheco FV, Alvarenga AA, Bertolucci SKV, Pinto JEB (2018) Growth and production of volatile compounds of yarrow (Achillea millefolium L.) under different irrigation depths. An Acad Bras Cienc 90:3901-3910. http://dx.doi.org/10.1590/0001-3765201820180092
Apostol I, Heinstein PF, Low OS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol 90:109-116. https://doi.org/10.1104/pp.90.1.109
Araújo MJD, Camara CAD, Moraes MM, Born FS, (2020) Insecticidal properties and chemical composition of Piper aduncum L., Lippia sidoides Cham. and Schinus terebinthifolius Raddi essential oils against Plutella xylostella L. An Acad Bras Cienc 92:supl. 1:e20180895,1-14. http://dx.doi.org/10.1590/0001-3765202020180895
Baghalian K, Haghiry A, Naghavi MR, Mohammadi A (2008) Effect of saline irrigation water on agronomical and phytochemical characters of chamomile (Matricaria recutita L.). Sci Hortic116:437-441. https://doi.org/10.1016/j.scienta.2008.02.014
Carvalho FEL, Lobo AKM, Bonifacio A, Martins MO, Lima Neto, MC, Silveira, JAG (2011) Aclimatação ao estresse salino em plantas de arroz induzida pelo pré-tratamento com H2O2. Rev Bras Eng Agr Amb 15:416–423. http://dx.doi.org/10.1590/S1415-43662011000400014
De Oliveira LM, Da Silva JN, Coelho CCR, Neves MG, Da Silva RTL, De Oliveira Neto CF (2013) Pigmentos fotossintetizantes, aminoácidos e proteínas em plantas jovens de graviola submetida ao déficit hídrico. Rev Agroecoss 5:39-44. http://dx.doi.org/10.18542/ragros.v5i1.1409
Falahi H, Sharifi M, Maivan HZ, Chashmi NA (2018) Phenylethanoid glycosides accumulation in roots of Scrophularia striata as a response to water stress. Environ Exp Bot 147:13-21. https://doi.org/10.1016/j.envexpbot.2017.11.003
Fazolin M, Estrela JL, Catani V, Lima MSD, Alécio MR. 2005. Toxicity of Piper aduncum oil to adults of Cerotoma tingomarianus Bechyné (Coleoptera: Chrysomelidae). Neotrop Entomol 34:485-489. http://dx.doi.org/10.1590/S1519-566X2005000300018
Fini A, Guidi L, Ferrini F, Brunetti C, Di Ferdinando M, Biricolti S, Pollastri S, Calamai L, Tattini, M (2012) Drought stress has contrasting effects on antioxidant enzymes activity and phenylpropanoid biosynthesis in Fraxinus ornus leaves: an excess light stress affair. J Plant Physiol 169:929-939. https://doi.org/10.1016/j.jplph.2012.02.014
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134-142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866-1875. https://doi.org/10.1105/tpc.105.033589
Fu J, Huang B (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105-114. https://doi.org/10.1016/S0098-8472(00)00084-8
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309-314. https://doi.org/10.1104/pp.59.2.309
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909-930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gobbo-Neto L, Lopes NP (2007) Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Quim Nova 30:374-381. http://dx.doi.org/10.1590/S0100-40422007000200026
Gonçalves S, Martins N, Romano A (2017) Physiological traits and oxidative stress markers during acclimatization of micropropagated plants from two endangered Plantago species: P. algarbiensis Samp. and P. almogravensis Franco. In Vitro Cell Dev Pl 53:249-255. https://doi.org/10.1007/s11627-017-9812-y
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450-455. https://doi.org/10.1104/pp.84.2.450
Jacinto ACP, Souza LPD, Nakamura AT, Carvalho FJ, Simão E, Zocoler JL, Bergo CL (2018) Idioblasts formation and essential oil production in irrigated Piper aduncum. Pesqui Agropec Trop 48:447-452. http://dx.doi.org/10.1590/1983-40632018v4853165
Lee H, Jeon J, Yoon J, Kim SH, Choi HS, Kang JS, Lee YS, Lee M, Kim YH, Chang HB. 2020. Comparative metabolite profiling of wild and cultivated Justicia procumbens L. based on 1H-NMR Spectroscopy and HPLC-DAD Analysis Plants 9:1-12. https://doi.org/10.3390/plants9070860
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. Curr Protoc Food Anal Chem Sup. F4.3.1–F4.3.8. https://doi.org/10.1002/0471142913.faf0403s01
Lima MDGDS, Lopes NF, Bacarin MA, Mendes CR (2004) Efeito do estresse salino sobre a concentração de pigmentos e prolina em folhas de arroz. Bragantia 63:335-340. http://dx.doi.org/10.1590/S0006-87052004000300003
Lopes OD, Kobayashi MK, Oliveira FG, Alvarenga ICA, Martins E R, Corsato CE (2011) Determinação do coeficiente de cultura (Kc) e eficiência do uso de água do alecrim-pimenta irrigado. Rev Bras Eng Agr Amb 15:548-554. http://dx.doi.org/10.1590/S1415-43662011000600002
Mafakheri A, Siosemardeh AF, Bahramnejad B, Struik, PC, SohrabI Y (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580-585.
Masoumi A, Kafi M, Khazaei H, Davari K (2010) Effect of drought stress on water status, elecrolyte leakage and enzymatic antioxidants of kochia (Kochia scoparia) under saline condition. Pakistan J Bot 42:3517-3524.
Meira MR, Melo MTPD, Martins ER, Pinto MJDS, Santana CS (2013) Crescimento vegetativo, produção de fitomassa e de óleo essencial de Melissa officinalis L. sob diferentes lâminas de irrigação. Cienc Rural 43:779-785. http://dx.doi.org/10.1590/S0103-84782013005000040.
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbatospecific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867-880. https://doi.org/10.1093/oxfordjournals.pcp.a076232.
Nist Speech Group Website. Topic detection and tracking evaluation (2008) Online. Available in:< http://webbook.nist.gov/chemistry> Acess in 15 fev. 2013.
Oliveira GL, Cardoso SK, Lara Junior CR, Vieira TM, Guimarães EF, Figueiredo LS, Martins ER, Moreira DL, Kaplan MAC (2013) Chemical study and larvicidal activity against Aedes aegypti of essential oil of Piper aduncum L.(Piperaceae). An Acad Bras Cienc 85:1227-1234. http://dx.doi.org/10.1590/0001-3765201391011
Pacheco F V, Avelar RP, Alvarenga ICA, Bertolucci SKV, De Alvarenga AA, Pinto, JEBP (2016) Essential oil of monkey-pepper (Piper aduncum L.) cultivated under different light environments. Ind Crop Prod 85:251-257. https://doi.org/10.1016/j.indcrop.2016.03.016
Parmar VS, Jain SC, Gupta S, Talwar S, Rajwanshi VK, Kumar R, Azim A, Malhotra S, Kumar N, Jain R, Sharma NK, Tyagi OD, Lawrie SJ, Errington W, Howarth OW, Olsen CE, Singh SK, Wengel J (1997) Phytochemistry of the genus Piper. Phytochem 46, 597-673. https://doi.org/10.1016/S0031-9422(97)00328-2
Perl-Treves R, Perl A (2002) Oxidative stress: an introduction. Oxidative stress in plants, p. 1-32, 2002.
Potzernheim MCL, Bizzo HR, Vieira RF (2006) Análise dos óleos essenciais de três espécies de Piper coletadas na região do Distrito Federal (Cerrado) e comparação com óleos de plantas procedentes da região de Paraty, RJ (Mata Atlântica). Rev Bras Farmacogn 16:246-251. https://doi.org/10.1590/S0102-695X2006000200019
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765-771. https://doi.org/10.1016/S0168-9452(01)00462-9
Rali T, Wossa SW, Leach DN, Waterman PG (2007) Volatile chemical constituents of Piper aduncum L. and . Piper gibbilimbum C. DC (Piperaceae) from Papua New Guinea. Molecules 12:389-394. https://doi.org/10.3390/12030389.
Rocha SF, Ming LC, Chaves FC, Scarda, FM. 2005. Role of light and phytochrome on Piper aduncum L. germination: an adaptive and environmental approach. J Herbs Spices Med Plants 11:85-96. https://doi.org/10.1300/J044v11n03_08
SAEG. Sistema para análise estatística, Versão 9.1.Viçosa: Fundação Arthur Bernardes da Universidade Federal de Viçosa (2007). Available in <http://arquivo.ufv.br/saeg>. Acess in 30 Ago. 2020.
Santos TT, Santos MF, Mendonça MC, Silva Júnior CD, Silva-Mann R, Arrigoni-Blank MDF, Blank AF (2004) Efeito do estresse hídrico na produção de massa foliar e teor de óleo essencial em sambacaitá (Hyptis pectinata L.). In: Congresso Brasileiro De Olericultura. Campo Grande: Congresso de Olericultura, 2004. p. 1-4.
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crop Prod 42:558-566. https://doi.org/10.1016/j.indcrop.2012.06.020
Shvaleva AL, Silva FCE, Breia E, Jouve J, Hausman JF, Almeida M H, Maroco JP, Rodrigues ML, Pereira JS, Chaves MM (2006) Metabolic responses to water deficit in two Eucalyptus globulus clones with contrasting drought sensitivity. Tree Physiol 26:239-248. https://doi.org/10.1093/treephys/26.2.239
Singh M, Khan MMA, Uddin M, Naeem M, Qureshi MI (2017) Proliferating effect of radiolytically depolymerized carrageenan on physiological attributes, plant water relation parameters, essential oil production and active constituents of Cymbopogon flexuosus Steud. under drought stress. Plos One 12:e0180129. https://doi.org/10.1371/journal.pone.0180129
Sousa PJ, Barros CA, Rocha JCS, Lira DS, Monteiro GM, Maia JGS (2008) Avaliação toxicológica do óleo essencial de Piper aduncum L. Rev Bras Farmacogn 18:217-221. https://doi.org/10.1590/S0102-695X2008000200013
Souza GS, Oliveira UC, Silva JS, Lima JC (2013) Crescimento, produção de biomassa e aspectos fisiológicos de plantas de Mentha piperita L. cultivadas sob diferentes doses de fósforo e malhas coloridas. Global Sci Tech 6. http://dx.doi.org/10.14688/1984-3801.v06n03a04
Streit NM, Canterle LP, Canto MWD, Hecktheuer LHH (2005) As clorofilas. Cienc Rural 35, 748-755. http://dx.doi.org/10.1590/S0103-84782005000300043
Taiz L, Zeiger E, Moller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal. 6.ed. Porto Alegre: Artmed.
Van Den Dool H, Kratz PDJA (1963) Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463-471. https://doi.org/10.1016/S0021-9673(01)80947-X
Vieira SCH, Paulo LFD, Svidzinski TIE, Dias Filho BP, Nakamura CV, Souza AD, Mark MC, Cortez DAG (2011) Antifungal activity of Piper diospyrifolium Kunth (Piperaceae) essential oil. Braz J Microbiol 42:1001-1006. http://dx.doi.org/10.1590/S1517-83822011000300020
Yang Z, Liu J, Poree F, Schaeufele R, Helmke H, FrackenpohL J, Lehr S, Koskull-Doring P, Christmann A, Schnyder H, Schmidhalter U, Grill E (2019) Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol 180:1066-1080. https://doi.org/10.1104/pp.18.01238
Yi XP, Zhang YL, Yao HS, Luo HH, Gou L, Chow WS, Zhang WF (2016) Rapid recovery of photosynthetic rate following soil water deficit and re-watering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. J Plant Physiol 194:23-34. https://doi.org/10.1016/j.jplph.2016.01.016
Yuncker TG (1975) The Piperaceae of Brazil. Hoehnea 2:99-105.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

